Summary of Recent Publication “Parylene Scaffold for Cartilage Lesion”
 Recent research performed by Carlos Eduardo da Silveira Franciozi, Dr. Vangsness, and colleagues identifies a potential new way of treating cartilage injuries. This study, published in April 2017 by the bimonthly scientific journal Biomedical Microdevices, presents their findings.
Recent research performed by Carlos Eduardo da Silveira Franciozi, Dr. Vangsness, and colleagues identifies a potential new way of treating cartilage injuries. This study, published in April 2017 by the bimonthly scientific journal Biomedical Microdevices, presents their findings.
Background
Injury to joint cartilage can result in pain, disability, joint degeneration, and arthritis. Current surgical treatments for cartilage lesions have a number of limitations. For this study, the authors adopted a procedure that combined reparative and superficial reconstructive techniques.
Parylene-C, a biocompatible polymer, has the potential to serve as a scaffold for cartilage lesion treatment. Before the study, parylene-C had never been tested as a cartilage scaffold, but it had been approved for chronic implantation into the body. Parylene has been used successfully in ophthalmic studies, and its mechanical strength and biostability make it a viable option for articular cartilage.
The Study
The study was humanely performed on 15 rabbits. A parylene scaffold was implanted over the right knee lesion, and the left knee served as a control. Imaging and immunostaining were used to assess the effectiveness of the treatment at various points (3, 6, 9, and 12 weeks).
Study Results
The knees that were given a parylene scaffold showed significantly better signs of repair than the control knees. These findings were consistent across all evaluation points. There was no inflammation, and the parylene-implanted knees had higher collagen expression, which suggests a better healing response.
Conclusions
The results of this study indicate that a parylene scaffold may be an option for treating cartilage lesions. In the study, a microfracture bone marrow stimulating technique (reparative) and the “protective cap” parylene scaffold (superficial reconstructive) shielded the tissue in a way that shows promising results. While these preliminary results are encouraging, further animal studies are needed to determine whether this treatment will provide benefits in humans.
Dr. Vangsness is our senior orthopaedic surgeon at USC. Dedicated to orthopaedic medicine, Dr. Vangsness works hard to identify the best ways to treat orthopaedic conditions and improve patients’ quality of life. To learn more about his research or to receive orthopaedic treatment, request your personal consultation today with Dr. Vangsness. Call (323) 442-5800 or contact us online to schedule an appointment.
Summary of Recent Publication, “Mesenchymal Stem Cell Levels of Human Spinal Tissues”
 Back pain caused by degenerative disc disease has been estimated to affect nearly 1 in 3 Americans and costs the United States roughly 50 to 100 billion dollars in lost work hours each year. Mesenchymal stem cells represent an exciting new development in the treatment of back pain. Dr. Vangsness and colleagues recently conducted a study of isolated mesenchymal stem cells to improve treatment for degenerative disc disease. Their findings suggest that human spinal tissue is a consistent and reliable source of these unique cells.
Back pain caused by degenerative disc disease has been estimated to affect nearly 1 in 3 Americans and costs the United States roughly 50 to 100 billion dollars in lost work hours each year. Mesenchymal stem cells represent an exciting new development in the treatment of back pain. Dr. Vangsness and colleagues recently conducted a study of isolated mesenchymal stem cells to improve treatment for degenerative disc disease. Their findings suggest that human spinal tissue is a consistent and reliable source of these unique cells.
Background
Current treatments for back pain are generally limited to analgesics, physical therapy, and surgery. While conservative measures such as analgesics and physical therapy can often improve back pain, surgical treatment for degenerative back pain is often ineffective. Consequently, new treatments to slow, prevent, and reverse degenerative spine disease are critical to improving the care and quality of life for back pain sufferers.
Mesenchymal stem cells have regenerative abilities that demonstrate a promising application in the treatment of degenerative conditions that have traditionally had poor prognoses. As the study of these cells has progressed, mesenchymal stem cells have been found and isolated from tissues throughout the body. Only recently, however, have mesenchymal stem cells been isolated from tissues within the spine itself, and prior to this study, their numbers within spinal tissues had yet to be examined. For these reasons, the authors of the study sought to compare the levels of mesenchymal stem cells isolated from human spine tissues to those from other tissues in the body.
The Study
The study was conducted as a review of the current literature available on the PubMed, MEDLINE, EMBASE, and Cochrane databases. The authors reviewed articles relating to the harvest, characterization, isolation, and quantification of human mesenchymal stem cells from spinal tissues. Selected articles were examined for relevant data, categorized according to the type of spinal tissue, and when possible, standardized to facilitate comparisons between sites.
Study Results
The results of the study showed that mesenchymal stem cells in various tissues of the spine were considerably higher than previously thought. Levels within the ligamentum flavum (a type of spinal tissue) were comparable to bone marrow, which is the traditional standard source for stem cell harvest. The study found that mesenchymal stem cells have been successfully isolated from five different tissues in all levels of the spine.
Conclusions
The results of the study present a favorable conclusion. Numerous tissues within and surrounding the spine represent a consistent and reliable source for the harvest and isolation of human mesenchymal stem cells. Among the tissues of the spine, the annulus fibrosus and ligamentum flavum each offer considerable levels of mesenchymal stem cells, which may prove comparable to that of bone marrow. In application, these mesenchymal stem cells show promise in improving effective treatments of back pain for individuals with degenerative disc disease.
Summary of Recent Publication “The Placenta: Applications in Orthopaedic Sports Medicine”
Orthopaedic surgeon Dr. C. Thomas Vangsness, Jr., places a high priority on identifying new and better methods of treatment in his field. Recently, Dr. Vangsness and colleagues published an article in American Journal of Sports Medicine in which they investigated the therapeutic potential for the use of placental tissue in orthopaedic sports medicine.
Background
Interest in the regenerative capabilities of stem cells has increased rapidly over the past two decades. This has been largely due to the discovery of MSCs. MSCs are often referred to as mesenchymal stem cells, but they are different from stem cells in important ways. While scientists are still trying to understand how MSCs work, recent research suggests that MSCs release chemical signals that promote repair.
The human placenta is a rich source of MSCs. In recent years, a number of groups have started to investigate the regenerative potential of placental MSCs for orthopaedic conditions.
Study Results
To date, placenta-derived products have been used to treat a number of different conditions, including tendon, cartilage, and ligament injuries as well as osteoarthritis and joint inflammation. However, most of these studies have been carried out in animals. The only human studies using placental tissues that have been conducted to date involve difficult skin/wound coverage situations, such as severe burns and diabetic ulcers.
Conclusions
Recent data suggest that placental tissues and placenta-derived products may have potential benefits. However, clinical use of these treatments has been limited. When considering placenta-based treatments, it is important to understand that the currently available placenta-derived treatments are not MSCs or stem cell treatments.
Dr. Vangsness is a highly dedicated senior orthopaedic surgeon at USC. If you would like to learn more about the research he has conducted or if you are in need of orthopaedic treatment, please request your consultation today. Call (323) 442-5800 or contact us online to schedule your appointment.
What Is a Meniscus Tear?
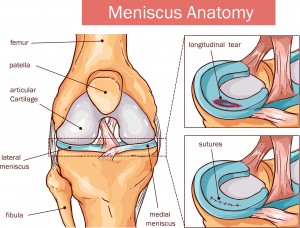
The meniscus is comprised of two wedges of cartilage (the medial and lateral meniscus), and it cushions the knee joint where the leg bones meet. Your meniscus acts as a shock absorber, allowing the knee to move smoothly. Damage to the meniscus is commonly seen in athletes with twisting injuries. A tear in the meniscus can cause pain and long-lasting damage. Correcting a meniscus tear as soon as possible will help improve your long-term use of the knee.
How Does a Meniscus Tear Occur?
 In athletes, a meniscus tear can occur because of a blow to the knee, over-rotation of the knee, planting a foot awkwardly, or hyperextension of the knee. This can happen in non-athletes as well. Other ways to develop a meniscus injury that aren’t directly related to sports include osteoarthritis, repetitive movements (such as squatting or kneeling), or acute (sudden) injury. In older patients, tears can sometimes develop over time with no known cause.
In athletes, a meniscus tear can occur because of a blow to the knee, over-rotation of the knee, planting a foot awkwardly, or hyperextension of the knee. This can happen in non-athletes as well. Other ways to develop a meniscus injury that aren’t directly related to sports include osteoarthritis, repetitive movements (such as squatting or kneeling), or acute (sudden) injury. In older patients, tears can sometimes develop over time with no known cause.
Symptoms of a Meniscus Tear
- An initial sharp pain or “popping” sensation
- Tenderness along the knee joint line
- Stiffness and/or swelling
- Clicking, popping, catching, or locking of the knee
- Pain when walking down stairs
Types of Meniscal Tears
- Vertical, “bucket-handle tear” – the meniscus tears lengthwise
- Caused when the femur and tibia trap the meniscus when the knee rotates
- Most common in young athletes
- Oblique, “flap tear” or “parrot beak tear” – the meniscus tears where the posterior and middle thirds of the meniscus meet
- Caused by repetitive stress such as running
- Horizontal, “transverse tear” – the inner meniscus margin tears peripherally
- Most common in older patients due to degenerative changes
How to Treat a Meniscus Tear
If you are experiencing any symptoms of a meniscus tear, immediately begin treating with RICE: rest, ice, compression, and elevation. Maintain this treatment for the first 48 hours to decrease pain and minimize inflammation. Ibuprofen or naproxen may be taken for pain management.
The majority of the meniscus has poor blood supply and won’t heal internally. This is why surgery is necessary in many situations. A minimally invasive technique, arthroscopic surgery, can be used to repair the meniscus. An arthroscope can visualize the inside of the knee, and other small tools can fix a torn meniscus by removing or trimming away the damaged portion of cartilage. Occasionally, repairs can be made using sutures or absorbable plastic arrows or staples.
In situations where the cartilage is extensively damaged, a meniscus transplantation may be necessary. However, a limited number of people qualify for this technique. Meniscal transplant candidates:
- Are physically active and under 55 years of age
- Are missing more than 50 percent of their meniscus
- Have persistent pain related to physical activity involving the knee
- Have minimal evidence of arthritis in the damaged knee
Dr. Vangsness is a leading senior orthopedic surgeon at USC. If you need a meniscus tear consultation or have any questions about a previous meniscal surgery, schedule your consultation today. Call us at (323) 442-5800 or contact us online.
Platelet-Rich Plasma (PRP)
Platelet-Rich Plasma is a product that is believed to have very strong anti-inflammatory properties. Studies have shown that PRP is most effective at treating tendonitis, including Achilles tendonitis, lateral epicondylitis (tennis elbow), and medial epicondylitis (golfer’s elbow). PRP has also seen some scientific success treating knee arthritis.
There are several commercial devices that create different PRP mixtures, but the basic method for making PRP is the same for every device. Your own blood is drawn into a tube. That tube is then centrifuged, which is a process that separates blood contents into visible layers. The top layer is the plasma, the middle layer (buffy coat) contains white blood cells (as well as anti-inflammatory and growth factors), and the bottom layer contains red blood cells. The plasma portion is removed and then the buffy coat is drawn into another tube and injected right away. PRP’s results are variable, especially in arthritis. PRP injections are not covered by insurance or Medicare.